What Makes an Innovator?
-
Patients, Caregivers and Society
Form the backbone for everything we are
trying to achieve as an industry
-
Efficient Regulatory Affairs
Ensure that the drugs are safe and effective, reach the people who need them across the globe.
-
Pharmaceutical R&D
Helps generate path-breaking innovator
drugs and originator molecules that ensure betterment of lives
Innovator Product Specific Services

- Regulatory strategy for drug development
- Technical consulting (CMC) on drug development issues
- Pre-IND/BPD/ Scientific advice meeting support
- Preparation of meeting requests, questions and background materials and justifications/ briefing packages
- Representation for Agency meetings
- Strategic support for post-meeting preparation activities

- IND/IMPD/ CTA dossier templates
- Source data review/ gap analysis and Regulatory compliance support
- Preparation of IND/IMPD/CTA packages
- Regulatory support to manage clinical hold issues and HA queries/ Information requests
- IND/IMPD/ CTA amendments
- Annual reporting (as applicable) for clinical programs
- End of clinical phase meetings with HA

- Source data gap analysis for NDA/BLA/MAA dossier preparation
- Regulatory risk mitigation/ remediation plans for identified gaps
- NDA/BLA/MAA dossier templates
- Preparation and submission of NDA/BLA/MAA/CSD applications
- Support in handling HA queries/ information request/ amendments during review of application
- Regulatory support to manage response to CR letters (Complete Response Letters)

- Regulatory support for strategy and planning of compliance for post approval commitments
- Change controls assessment, Regulatory impact evaluation
- Regulatory strategy for filing the variations/ supplements in global market
- Preparation and submission of variation/ supplements packages to Health Authorities
- Preparation of response to queries received from the Agencies
- Annual reports submission
- Administrative submissions such as License Holder transfer, MA holder address changes
Freyr is Probably the First and the Only Global Regulatory Consulting Firm to Say…
Forget Regulatory Affairs
The pharmaceutical innovation model is remarkably robust and MNCs, small companies and virtual companies alike, have been very successful in maintaining their market dominance over the years owing to a strong NME pipeline and focus on R&D.
Which is why we urge you, to…
Focus on Innovation
Countries with a highly demanding Regulatory apparatus, like the United States and the UK, have promulgated an era of a competitive and innovative Pharmaceutical industry. This is in lieu of their stringent Regulatory requirements that in turn ensure that companies are more selective in the molecules that they bring to market.
With a strong global team of 800+ Regulatory affairs experts, and a combined millennium and more of experience, we are poised to, and we are confident that…
We’ll Handle Your Regulatory
- 01Do you have a unique drug technology that was never approved by Health Authorities before?
- 02Are you developing drugs for rare diseases?
- 03Are you exploring new therapeutic areas with novel drug candidates?
- 04Are you looking for the right strategy for your drug development and global commercialization?
- 05Are you a company that has purchased a molecule still in clinical trials?
- 06Are you a company that has a long pipeline of innovators?
- 07Are you a virtual company looking to expand into multiple global market?
If your answer to any of these is “yes”…
If you do not have an in-house Regulatory affairs team …
If your in-house team needs support in terms of global expertise and resources …
Come Talk to Us
From the Eureka moment to the actual filing, and even after that (it's not over till it actually is) Freyr acts as a strategic Regulatory partner providing end-to-end Regulatory solutions and services.
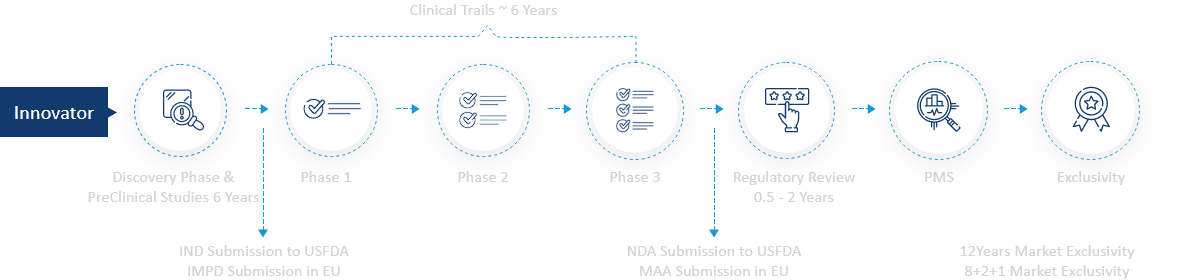
The Innovator Drug Development Process
Clinical Trials ~ 6 Years



Discovery Phase & PreClinical Studies 6 Years


Phase 1


Phase 2


Phase 3

Regulatory Review
0.5 - 2 Years

PMS


Exclusivity



- IND Submission to USFDA
IMPD Submission in EU - NDA Submission to USFDA
MAA Submission in EU - 12Years Market Exclusivity
8+2+1 Market Exclusivity
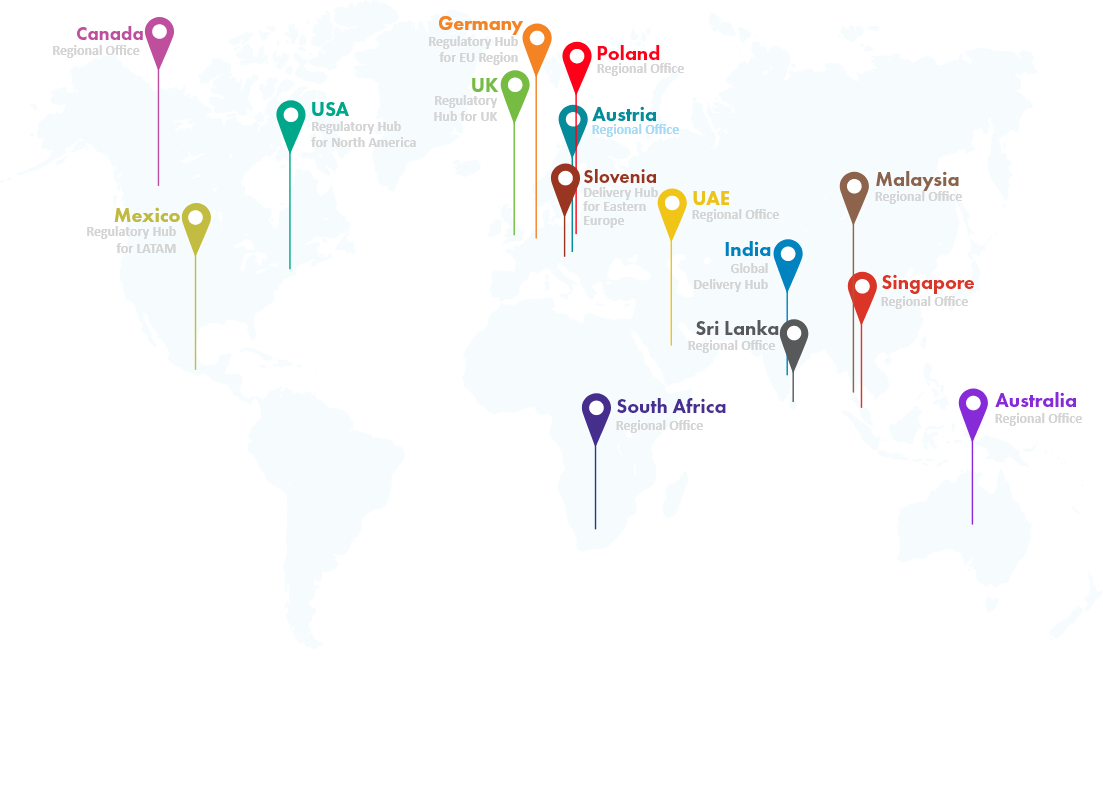
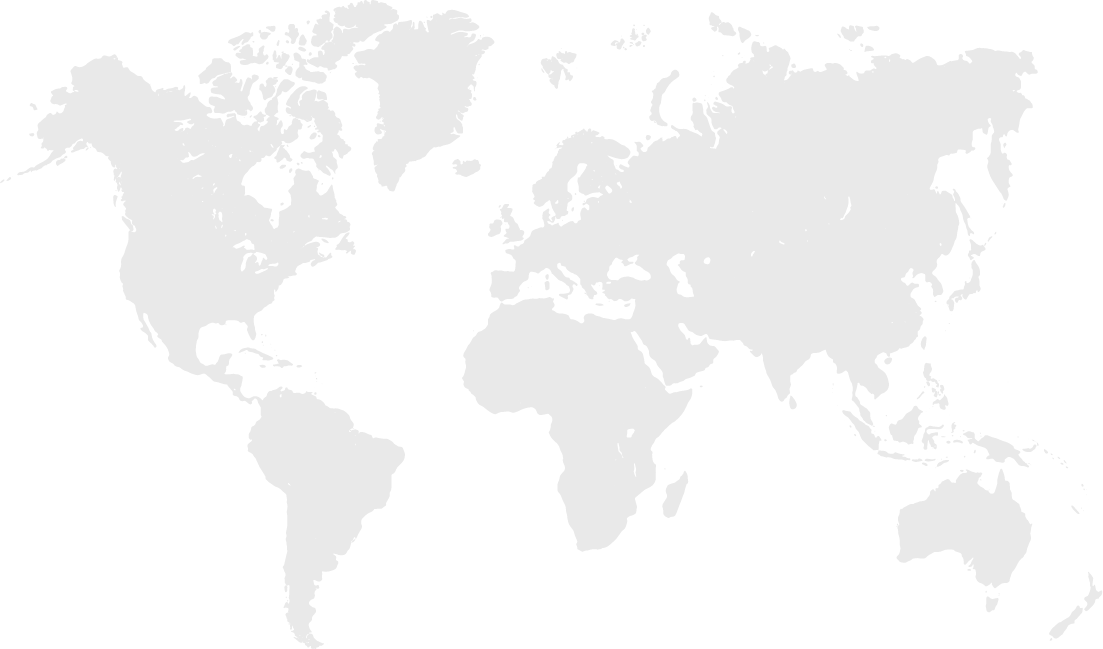
Global Locations
 120+ Countries
120+ Countries- &
 13+ Regulatory Hubs
13+ Regulatory Hubs
-
Canada
Regional Office -
Mexico
Regulatory Hub
for LATAM -
USA
Regulatory Hub
for North America -
Germany
Regulatory Hub
for EU Region -
UK
Regulatory
Hub for UK -
Poland
Regional Office -
Slovenia
Delivery Hub for Eastern Europe -
India
Global Delivery Hub -
Sri Lanka
Regional Office -
South Africa
Regional Office -
Malaysia
Regional Office -
Singapore
Regional Office -
Australia
Regional Office
Regulatory Competencies
Compliance, Audit And Validation
- GxP Audit Services
- QMS Remediation Services
- Process and Product Validation
- GxP Solution Consulting
- Clinical Trial Audit and Monitoring Services

-
0+
Clients
-
0+
Innovator Projects -
0+
Annual Reports -
0K+
Post Approval
Activities -
0K+
Submission -
0k+
Labels Processed
Annually -
0+
NDAs(505(b)(1)) -
0+
MAAs(Article 8(3)) -
0+
Regulatory partner
for development of
120+ Drug Candidates -
0+
INDs -
0+
CTA -
0+
Pre-Submission
meetings -
0+
Scientific Advice
Meetings -
0K+
Renewals
meetings